Gram staining
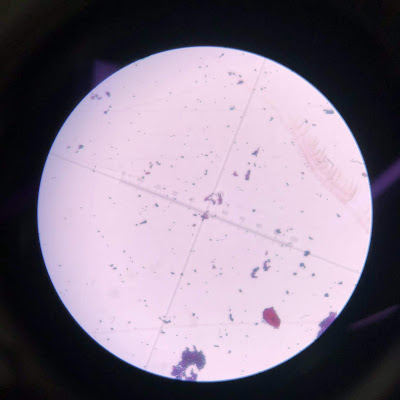
Happy sunny morning!!! I came the busy lab in Saturday morning and did few gram staining experiments for caeni 2 and caeni 3. Both of them are Gram positive bacteria. Gram positive bacteria: Dark purple Caeni 3 Gram positive bacteria : Dark purple Caeni 2





